-
Having a
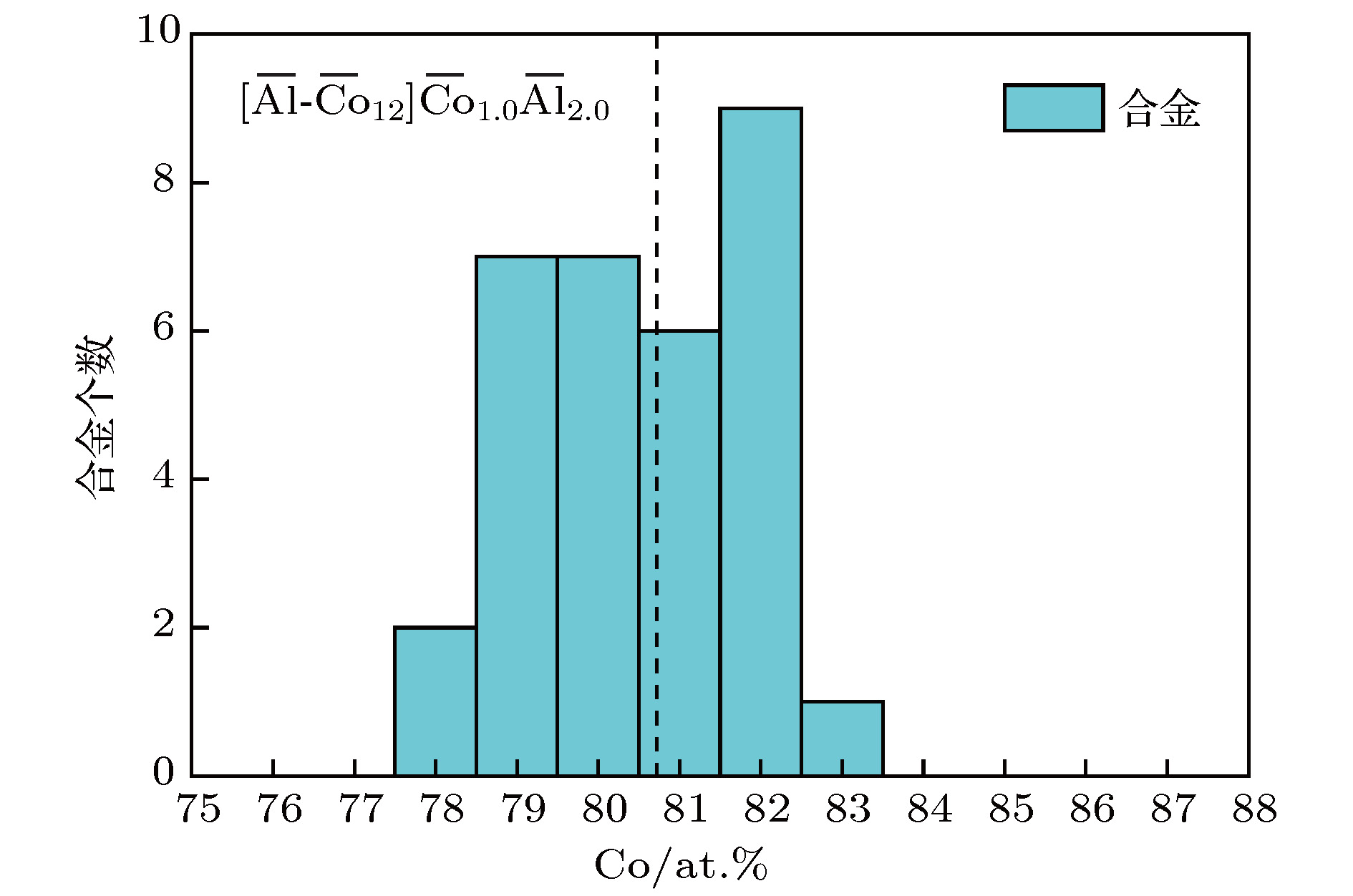
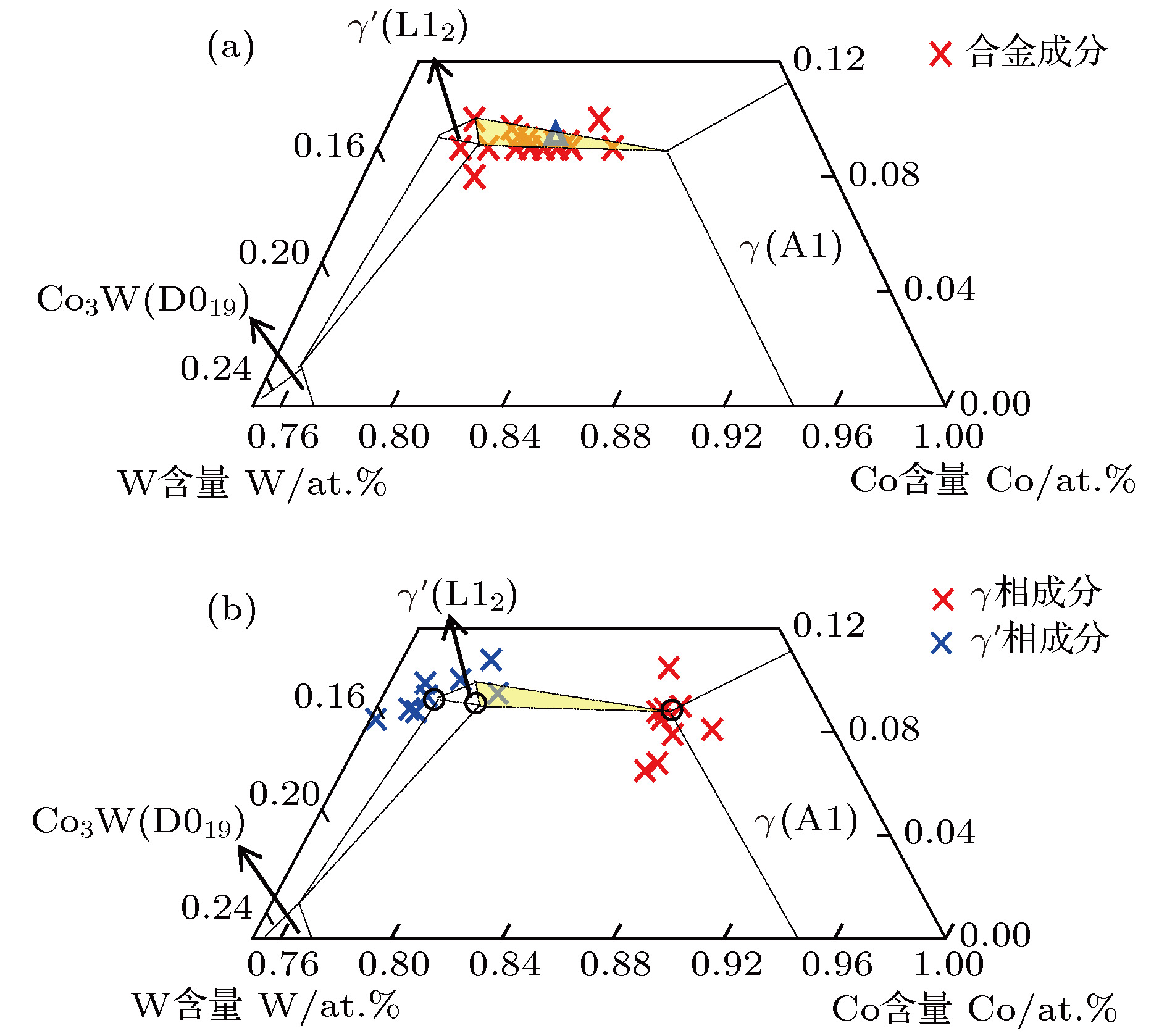
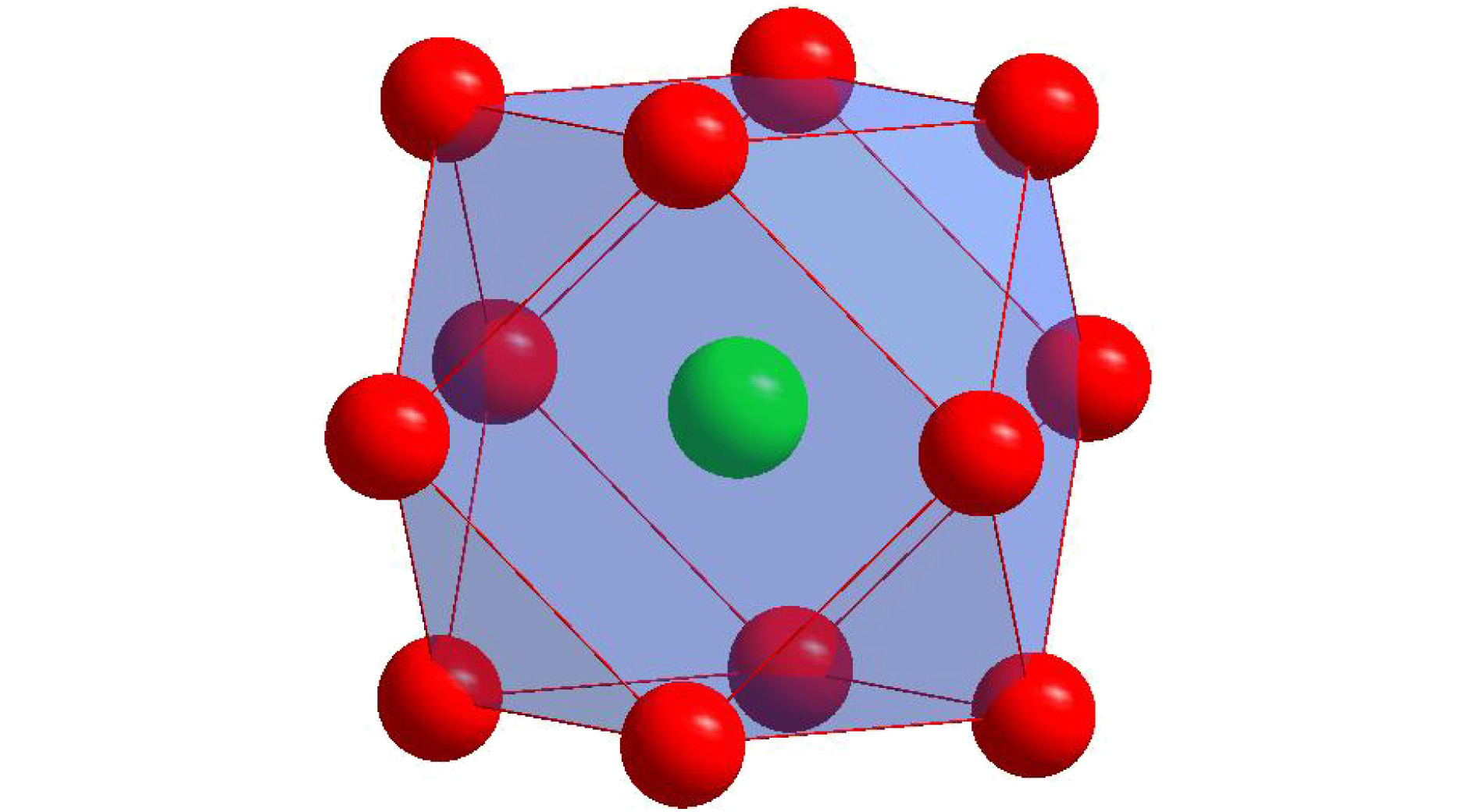
$\gamma /\gamma′ $ microstructure similar to Ni-base superalloys and also including various alloying elements such as Al and W, new Co-base superalloy, namely Co-Al-W-base alloy, has been widely studied as a kind of potential alternative of Ni-base superalloy, which is the most important high-temperature structural material in industrial applications. Besides, Co-Al-W-base alloy has also excellent mechanical properties, for example, creep properties comparable to those of the first-generation Ni-base single crystal superalloys. In our previous work, the ideal composition formula of Ni-base superalloy has been obtained by applying the cluster-plus-glue-atom structure model of faced centered cubic solid solution, which shows that the most stable chemical short-range-order unit is composed of a nearest-neighbor cluster and three next-neighbor glue atoms. In this paper, the ideal cluster formula of Co-Al-W-base superalloy is addressed by using the same approach. Based on cluster-plus-glue-atom model theory, according to lattice constants and atom radii, calculations are carried out. The results show that the atom radius of Al is equal to Covalent radius (0.126 nm) and for$\gamma′ $ phase the atom radius of W changes obviously (0.1316 nm). After analyzing atomic radii, the chemical formula for Co-Al-W ternary alloy is calculated to be [Al-Co 12](Co,Al,W) 3, which signifies an Al centered atom and twelve Co nearest-neighbored cluster atoms plus three glue atoms, which is in good consistence with that for Ni-base single crystal superalloy. For multi-element alloy, the alloying elements are classified, according to the heat of mixing between the alloying elements and Co as well as partition behavior of alloying elements, as solvent elements-Co-like elements$\overline {{\rm{Co}}} $ (Co, Ni, Ir, Ru, Cr, Fe, and Re) and solute elements-Al-like elements$\overline {{\rm{Al}}} $ (Al, W, Mo, Ta, Ti, Nb, V, etc.). The solvent elements can be divided into two kinds according to partition behaves:${\overline {{\rm{Co}}} ^{\gamma }}$ (Cr, Fe, and Re) and${\overline {{\rm{Co}}} ^{\gamma′}}$ (Ni, Ir, and Ru). The latter is further grouped into Al,${\overline {\rm{W}} }$ (W and Mo, which have weaker heat of mixing than Al-Co ) and${\overline {{\rm{Ta}}} }$ (Ta, Ti, Nb, V, etc., which have stronger heat of mixing than Al-Co). Then all chemically complex Co-Al-W-base superalloys are simplified into$\overline {{\rm{Co}}} \text{-} \overline {{\rm{Al}}} $ pseudo-binary or$\overline {{\rm{Co}}} \text{-} {\rm{Al}} \text{-} \left( {\overline {\rm{W}},\overline {{\rm{Ta}}} } \right)$ pseudo-ternary system. Within the framework of the cluster-plus-glue-atom formulism and by analyzing the compositions of alloy, it is shown that the Co-Al-W-base superalloy satisfies the ideal formula$\left[ {\overline {{\rm{Al}}} \text{-} {{\overline {{\rm{Co}}} }_{12}}} \right]\left( {{{\overline {{\rm{Co}}} }_{1.0}}{{\overline {{\rm{Al}}} }_{2.0}}} \right)$ (or$\left[ {{\rm{Al}} \text{-} {{\overline {{\rm{Co}}} }_{12}}} \right]{\overline {{\rm{Co}}} _{1.0}}{\rm{A}}{{\rm{l}}_{0.5}}{\left( {\overline {\rm{W}},\overline {{\rm{Ta}}} } \right)_{1.5}}$ =${\overline {{\rm{Co}}} _{81.250}}{\rm{A}}{{\rm{l}}_{9.375}}{\left( {\overline {\rm{W}},\overline {{\rm{Ta}}} } \right)_{9.375}}$ at.%). In the same way, those of$\gamma $ and$\gamma′ $ phases are respectively$\left[ {\overline {{\rm{Al}}} \text{-} {{\overline {{\rm{Co}}} }_{12}}} \right]\left( {{{\overline {{\rm{Co}}} }_{1.5}}{{\overline {{\rm{Al}}} }_{1.5}}} \right)$ (or$\left[ {{\rm{Al}} \text{-} {{\overline {{\rm{Co}}} }_{12}}} \right]{\overline {{\rm{Co}}} _{1.5}}{\rm{A}}{{\rm{l}}_{0.5}}{\left( {\overline {\rm{W}},\overline {{\rm{Ta}}} } \right)_{1.0}}$ =${\overline {{\rm{Co}}} _{84.375}}{\rm{A}}{{\rm{l}}_{9.375}}{\left( {\overline {\rm{W}},\overline {{\rm{Ta}}} } \right)_{6.250}}$ at.%) and$\left[ {\overline {{\rm{Al}}} \text{-} {{\overline {{\rm{Co}}} }_{12}}} \right]\left( {{{\overline {{\rm{Co}}} }_{0.5}}{{\overline {{\rm{Al}}} }_{2.5}}} \right)$ (or$\left[ {{\rm{Al}} \text{-} {{\overline {{\rm{Co}}} }_{12}}} \right]{\overline {{\rm{Co}}} _{0.5}}{\rm{A}}{{\rm{l}}_{0.5}}{\left( {\overline {\rm{W}},\overline {{\rm{Ta}}} } \right)_{2.0}}$ =${\overline {{\rm{Co}}} _{78.125}}{\rm{A}}{{\rm{l}}_{9.375}}{\left( {\overline {\rm{W}},\overline {{\rm{Ta}}} } \right)_{12.500}}$ at.%). For example, alloy Co 82Al 9W 9and its$\gamma $ and$\gamma′ $ phases are formulated respectively as [Al-Co 12]Co 1.1Al 0.4W 1.4(~ [Al-Co 12]Co 1.0Al 0.5W 1.5), [Al-Co 12]Co 1.6Al 0.4W 1.0(~ [Al-Co 12]Co 1.5Al 0.5W 1.0), and [Al-Co 12]Co 0.3Al 0.5W 2.2(~[Al-Co 12]Co 0.5Al 0.5W 2.0).-
Keywords:
- Co-Al-W-base superalloys/
- composition formula/
- cluster-plus-glue-atom model/
- chemical short-range order
[1] [2] [3] [4] [5] [6] [7] [8] [9] [10] [11] [12] [13] [14] [15] [16] [17] [18] [19] [20] [21] [22] [23] [24] [25] [26] [27] [28] [29] [30] [31] [32] [33] [34] [35] [36] [37] [38] [39] [40] [41] [42] [43] [44] [45] [46] [47] [48] [49] [50] [51] [52] [53] [54] [55] [56] [57] [58] [59] [60] [61] [62] -
合金成分/at.% $\gamma $相成分/at.% 晶格常数实验值/nm 晶格常数计算值/nm 绝对误差$\varDelta $ Co82Al9W9 Co81.7Al9.3W9 0.3580 0.3579 0.0001 Co83Al9W8 Co81.9Al10.0W8.1 0.3576 0.3575 0.0001 Co80Al9W11 Co80.7Al9.2W10.2 0.3586 0.3588 0.0002 Co74Al9W9Cr8 Co73.9Al8.0W6.8Cr11.2 0.3578 0.3575 0.0003 Co64Al9W9Ni18 Co69.1Al6.8W7.0Ni16.9 0.3577 0.3562 0.0015 Co65Al9W9Ni9Cr8 Co66.7Al7.8W6.7Ni8.3Cr10.7 0.3581 0.3584 0.0003 Co56Al9W9Ni18Cr8 Co59.2Al6.0W7.4Ni15.6Cr11.8 0.3583 0.3581 0.0002 Co72.5Ni10Al10W7.5 Co76.2Al8.7W5.4Ni9.7 0.3578 0.3562 0.0016 合金成分/at.% $\gamma′ $相成分/at.% 晶格常数实验值/nm W原子半径/nm Co82Al9W9 Co77.49Al10.03W12.48 0.3594 0.1317 Co83Al9W8 Co76.6Al9.4W14 0.3589 0.1306 Co80Al9W11 Co75.1Al9.1W15.8 0.3595 0.1311 Co74Al9W9Cr8 Co73.9Al9.4W10.4Cr6.3 0.3587 0.1314 Co64Al9W9Ni18 Co58.9Al10.8W11.0Ni19.3 0.3590 0.1317 Co65Al9W9Ni9Cr8 Co64.2Al10.1W9.9Ni9.4Cr6.4 0.3587 0.1317 Co56Al9W9Ni18Cr8 Co54.5Al10.5W9.7Ni19.7Cr5.6 0.3587 0.1319 Co72.5Ni10Al10W7.5 Co68.8Al10.8W9.9Ni10.5 0.3593 0.1324 元素
分类合金化
元素混合焓
$\Delta H$/kJ·mol元素配分
系数K${\overline {{\rm{Co}}} ^{\gamma }}$ Cr –4 0.48—0.60 Fe –1 Re 2 ${\overline {{\rm{Co}}} ^{\gamma′ }}$ Ni –2 1.08—1.27 Ru –1 Ir –3 Al Al –19 0.93—1.60 ${\overline {\rm{W}} }$ W –1 1.03—6.21 Mo –5 ${\overline {{\rm{Ta}}} }$ V –14 1.57—8.67 Ta –24 Nb –25 Ti –28 Sc –30 Hf –35 合金成分/at.% 团簇成分式-[团簇](连接原子)3 连接原子 Co78Al10W10Ta2 [Al-Co12]Co0.5Al0.6W1.6Ta0.3 ${\overline {{\rm{Co}}} ^{\gamma′ }}_{0.5}{\rm{A}}{{\rm{l}}_{0.6}}{\overline {\rm{W}} _{1.6}}{\overline {{\rm{Ta}}} _{0.3}}$ Co78Al9W10Mo3 [Al-Co12]Co0.5Al0.4W1.6Mo0.5 ${\overline {{\rm{Co}}} ^{\gamma′ }}_{0.5}{\rm{A}}{{\rm{l}}_{0.4}}{\overline {\rm{W}} _{2.1}}$ Co79Al9W10Ti2 [Al-Co12]Co0.6Al0.4W1.6Ti0.3 ${\overline {{\rm{Co}}} ^{\gamma′ }}_{0.6}{\rm{A}}{{\rm{l}}_{0.4}}{\overline {\rm{W}} _{1.6}}{\overline {{\rm{Ta}}} _{0.3}}$ Co79Al9W10V2 [Al-Co12]Co0.6Al0.4W1.6V0.3 ${\overline {{\rm{Co}}} ^{\gamma′ }}_{0.6}{\rm{A}}{{\rm{l}}_{0.4}}{\overline {\rm{W}} _{1.6}}{\overline {{\rm{Ta}}} _{0.3}}$ Co79Al9W10Si2 [Al-Co12]Co0.6Al0.4W1.6Si0.3 ${\overline {{\rm{Co}}} ^{\gamma′ }}_{0.6}{\rm{A}}{{\rm{l}}_{0.4}}{\overline {\rm{W}} _{1.6}}{\overline {{\rm{Ta}}} _{0.3}}$ Co79Al9W8Ta2Nb2 [Al-Co12]Co0.6Al0.4W1.3Ta0.3Nb0.3 ${\overline {{\rm{Co}}} ^{\gamma′ }}_{0.6}{\rm{A}}{{\rm{l}}_{0.4}}{\overline {\rm{W}} _{1.3}}{\overline {{\rm{Ta}}} _{0.6}}$ Co79Al9W8Ta2V2 [Al-Co12]Co0.6Al0.4W1.3Ta0.3V0.3 ${\overline {{\rm{Co}}} ^{\gamma′ }}_{0.6}{\rm{A}}{{\rm{l}}_{0.4}}{\overline {\rm{W}} _{1.3}}{\overline {{\rm{Ta}}} _{0.6}}$ Co79Al8W9Ta2Ti2 [Al-Co12]Co0.6Al0.3W1.4Ta0.3Ti0.3 ${\overline {{\rm{Co}}} ^{\gamma′ }}_{0.6}{\rm{A}}{{\rm{l}}_{0.3}}{\overline {\rm{W}} _{1.4}}{\overline {{\rm{Ta}}} _{0.6}}$ Co79.5Al9.7W10.8 [Al-Co12]Co0.7Al0.6W1.7 ${\overline {{\rm{Co}}} ^{\gamma′ }}_{0.7}{\rm{A}}{{\rm{l}}_{0.6}}{\overline {\rm{W}} _{1.7}}$ Co79.9Al9.4W10.7 [Al-Co12]Co0.8Al0.5W1.7 ${\overline {{\rm{Co}}} ^{\gamma′ }}_{0.8}{\rm{A}}{{\rm{l}}_{0.5}}{\overline {\rm{W}} _{1.7}}$ Co80Al9W11 [Al-Co12]Co0.8Al0.4W1.8 ${\overline {{\rm{Co}}} ^{\gamma′ }}_{0.8}{\rm{A}}{{\rm{l}}_{0.4}}{\overline {\rm{W}} _{1.8}}$ Co80Al9W9Ti2 [Al-Co12]Co0.8Al0.4W1.4Ti0.3 ${\overline {{\rm{Co}}} ^{\gamma′ }}_{0.8}{\rm{A}}{{\rm{l}}_{0.4}}{\overline {\rm{W}} _{1.4}}{\overline {{\rm{Ta}}} _{0.3}}$ Co80Al9W9V2B0.04 [Al-Co12]Co0.8Al0.4W1.4V0.3 ${\overline {{\rm{Co}}} ^{\gamma′ }}_{0.8}{\rm{A}}{{\rm{l}}_{0.4}}{\overline {\rm{W}} _{1.4}}{\overline {{\rm{Ta}}} _{0.3}}$ Co80Al9W9Ta2 [Al-Co12]Co0.8Al0.4W1.4Ta0.3 ${\overline {{\rm{Co}}} ^{\gamma′ }}_{0.8}{\rm{A}}{{\rm{l}}_{0.4}}{\overline {\rm{W}} _{1.4}}{\overline {{\rm{Ta}}} _{0.3}}$ Co80.3Al9.3W10.4 [Al-Co12]Co0.8Al0.5W1.7 ${\overline {{\rm{Co}}} ^{\gamma′ }}_{0.8}{\rm{A}}{{\rm{l}}_{0.5}}{\overline {\rm{W}} _{1.7}}$ Co80.5Al9W10Si0.5 [Al-Co12]Co0.9Al0.4W1.6Si0.1 ${\overline {{\rm{Co}}} ^{\gamma′ }}_{0.9}{\rm{A}}{{\rm{l}}_{0.4}}{\overline {\rm{W}} _{1.6}}{\overline {{\rm{Ta}}} _{0.1}}$ Co81Al9W9Mo1B0.04 [Al-Co12]Co1.0Al0.4W1.4Mo0.2 ${\overline {{\rm{Co}}} ^{\gamma′ }}_{1.0}{\rm{A}}{{\rm{l}}_{0.4}}{\overline {\rm{W}} _{1.6}}$ Co81Al9W8Ta2 [Al-Co12]Co1.0Al0.4W1.3Ta0.3 ${\overline {{\rm{Co}}} ^{\gamma′ }}_{1.0}{\rm{A}}{{\rm{l}}_{0.4}}{\overline {\rm{W}} _{1.3}}{\overline {{\rm{Ta}}} _{0.3}}$ Co81.3Al9.2W9.5 [Al-Co12]Co1.0Al0.5W1.5 ${\overline {{\rm{Co}}} ^{\gamma′ }}_{1.0}{\rm{A}}{{\rm{l}}_{0.5}}{\overline {\rm{W}} _{1.5}}$ Co81.5Al9W9Nb0.5 [Al-Co12]Co1.0Al0.4W1.4Nb0.1 ${\overline {{\rm{Co}}} ^{\gamma′ }}_{1.0}{\rm{A}}{{\rm{l}}_{0.4}}{\overline {\rm{W}} _{1.4}}{\overline {{\rm{Ta}}} _{0.1}}$ Co81.5Al9W5.5Ta2Mo2 [Al-Co12]Co1.0Al0.4W0.9Ta0.3Mo0.3 ${\overline {{\rm{Co}}} ^{\gamma′ }}_{1.0}{\rm{A}}{{\rm{l}}_{0.4}}{\overline {\rm{W}} _{1.2}}{\overline {{\rm{Ta}}} _{0.3}}$ Co82Al9W9 [Al-Co12]Co1.1Al0.4W1.4 ${\overline {{\rm{Co}}} ^{\gamma′ }}_{1.1}{\rm{A}}{{\rm{l}}_{0.4}}{\overline {\rm{W}} _{1.4}}$ Co72Al9W9Ni10 [Al-Co11.7Ni0.3]Ni1.1Al0.4W1.4 ${\overline {{\rm{Co}}} ^{\gamma′ }}_{1.1}{\rm{A}}{{\rm{l}}_{0.4}}{\overline {\rm{W}} _{1.4}}$ Co82Al9W7.5Mo1.5 [Al-Co12]Co1.1Al0.4W1.4 ${\overline {{\rm{Co}}} ^{\gamma′ }}_{1.1}{\rm{A}}{{\rm{l}}_{0.4}}{\overline {\rm{W}} _{1.4}}$ Co80Al9W9Cr2B0.04 [Al-Co12]Co0.8Cr0.3Al0.4W1.4 ${\overline {{\rm{Co}}} ^{\gamma′ }}_{0.8}{\overline {{\rm{Co}}} ^\gamma }_{0.3}{\rm{A}}{{\rm{l}}_{0.4}}{\overline {\rm{W}} _{1.6}}$ Co78Al9W9Cr4 [Al-Co12]Co0.6Cr0.6Al0.4W1.4 ${\overline {{\rm{Co}}} ^{\gamma′ }}_{0.6}{\overline {{\rm{Co}}} ^\gamma }_{0.6}{\rm{A}}{{\rm{l}}_{0.4}}{\overline {\rm{W}} _{1.4}}$ Co73Al9W9Ni9 [Al-Co11.7Ni0.3]Ni1.1Al0.4W1.4 ${\overline {{\rm{Co}}} ^{\gamma′ }}_{1.1}{\rm{A}}{{\rm{l}}_{0.4}}{\overline {\rm{W}} _{1.4}}$ Co64Al9W9Ni18 [Al-Co10.2Ni1.8]Ni1.1Al0.4W1.4 ${\overline {{\rm{Co}}} ^{\gamma′ }}_{1.1}{\rm{A}}{{\rm{l}}_{0.4}}{\overline {\rm{W}} _{1.4}}$ Co81.8Al9.2W9 [Al-Co12]Co1.1Al0.5W1.4 ${\overline {{\rm{Co}}} ^{\gamma′ }}_{1.1}{\rm{A}}{{\rm{l}}_{0.5}}{\overline {\rm{W}} _{1.4}}$ Co72.5Al10W7.5Ni10 [Al-Co11.6Ni0.4]Ni1.2Al0.4W1.4 ${\overline {{\rm{Co}}} ^{\gamma′ }}_{1.2}{\rm{A}}{{\rm{l}}_{0.4}}{\overline {\rm{W}} _{1.4}}$ Co81.5Al9W5.5Ta2Ir2 [Al-Co2]Co1.0Al0.4W0.9Ta0.3Ir0.3 ${\overline {{\rm{Co}}} ^{\gamma′ }}_{1.3}{\rm{A}}{{\rm{l}}_{0.4}}{\overline {\rm{W}} _{0.9}}{\overline {{\rm{Ta}}} _{0.3}}$ Co79Al9W8Ta2Cr2 [Al-Co12]Co0.6Cr0.3Al0.4W1.3Ta0.3 ${\overline {{\rm{Co}}} ^{\gamma′ }}_{0.6}{\overline {{\rm{Co}}} ^\gamma }_{0.3}{\rm{A}}{{\rm{l}}_{0.4}}{\overline {\rm{W}} _{1.3}}{\overline {{\rm{Ta}}} _{0.3}}$ 合金成分/at.% $\gamma $相团簇成分式 $\gamma′ $相团簇成分式 Co82Al9W9 [Al-Co12]Co1.6Al0.4W1.0 [Al-Co12]Co0.3Al0.5W2.2 Co78Al9W9Cr4 [Al-Co12]Co0.9Al0.3W0.9Cr0.9 [Al-Co12]Co0.2Al0.5W1.8Cr0.5 Co73Al9W9Ni18 [Al-Co11.1Ni0.9]Al0.1W1.1Ni1.8 [Al-Co9.4Ni2.6]Al0.7W1.8Ni0.5 Co79.5Al9.7W10.8 [Al-Co12]Co1.7Al0.4W0..9 [Al-Co12]Co0.4Al0.6W2.0 Co80Al9W9Ti2 [Al-Co12]Co1.6Al0.4W0.8Ti0.2 [Al-Co12]Co0.2Al0.4W1.9Ti0.4 Co80Al9W9Ta2 [Al-Co12]Co1.8Al0.4W0.7Ta0.1 [Al-Co12]Co0.2Al0.4W1.9Ta0.5 Co79Al8W9Ta2Ti2 [Al-Co12]Co2.0Al0.3W0.5Ta0.04Ti0.1 [Al-Co12]Co0.1Al0.4W1.9Ta0.3Ti0.3 Co78Al10W10Ta2 [Al-Co12]Co1.6Al0.7W0.7Ta0.1 [Al-Co12]Al0.7W1.9Ta0.4 Co78Al9W10Mo3 [Al-Co12]Co1.7Al0.1W0.8Mo0.4 [Al-Co12]Co0.2Al0.6W1.7Mo0.5 -
[1] [2] [3] [4] [5] [6] [7] [8] [9] [10] [11] [12] [13] [14] [15] [16] [17] [18] [19] [20] [21] [22] [23] [24] [25] [26] [27] [28] [29] [30] [31] [32] [33] [34] [35] [36] [37] [38] [39] [40] [41] [42] [43] [44] [45] [46] [47] [48] [49] [50] [51] [52] [53] [54] [55] [56] [57] [58] [59] [60] [61] [62]
Catalog
Metrics
- Abstract views:8203
- PDF Downloads:64
- Cited By:0


































 DownLoad:
DownLoad: