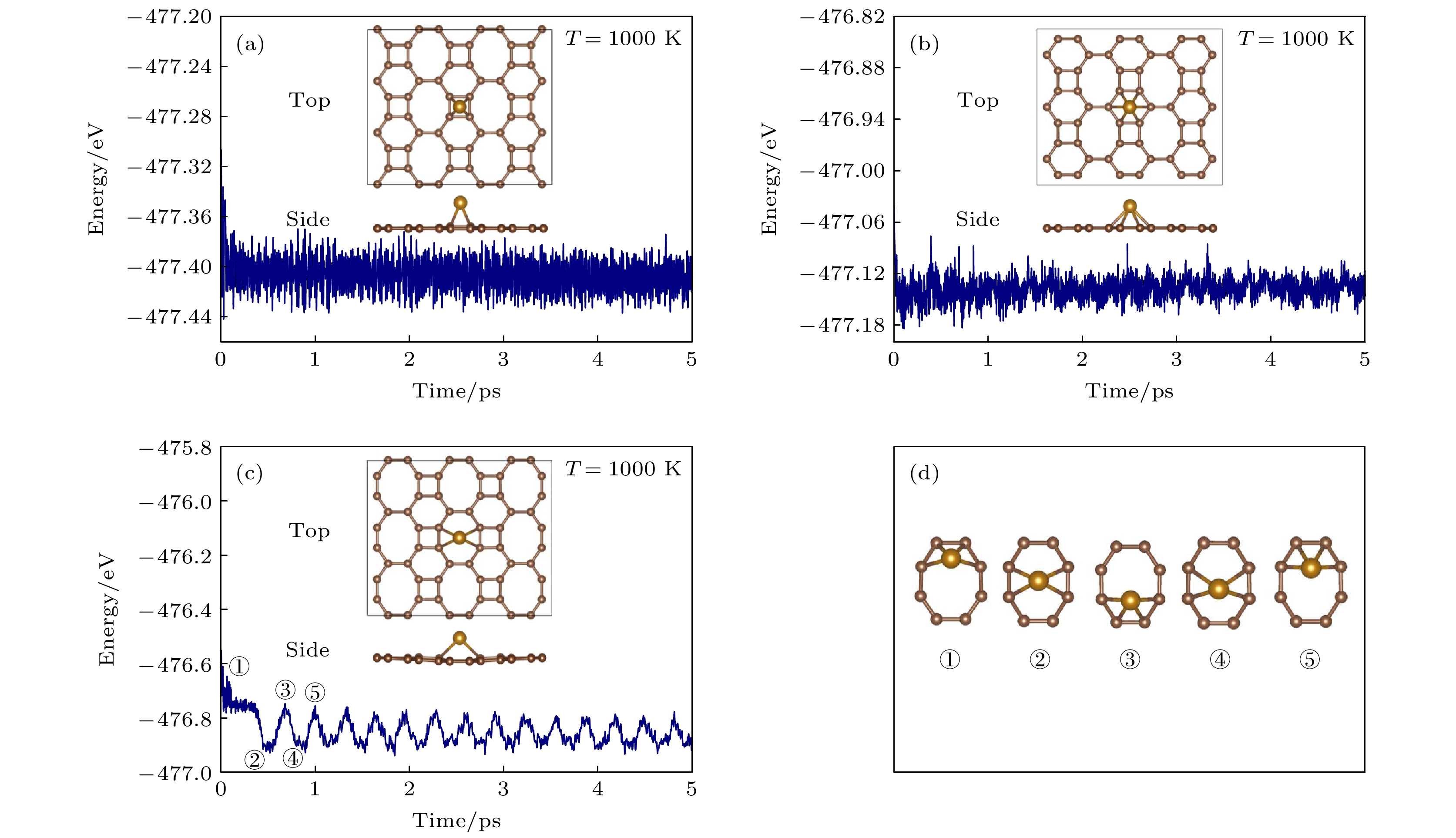
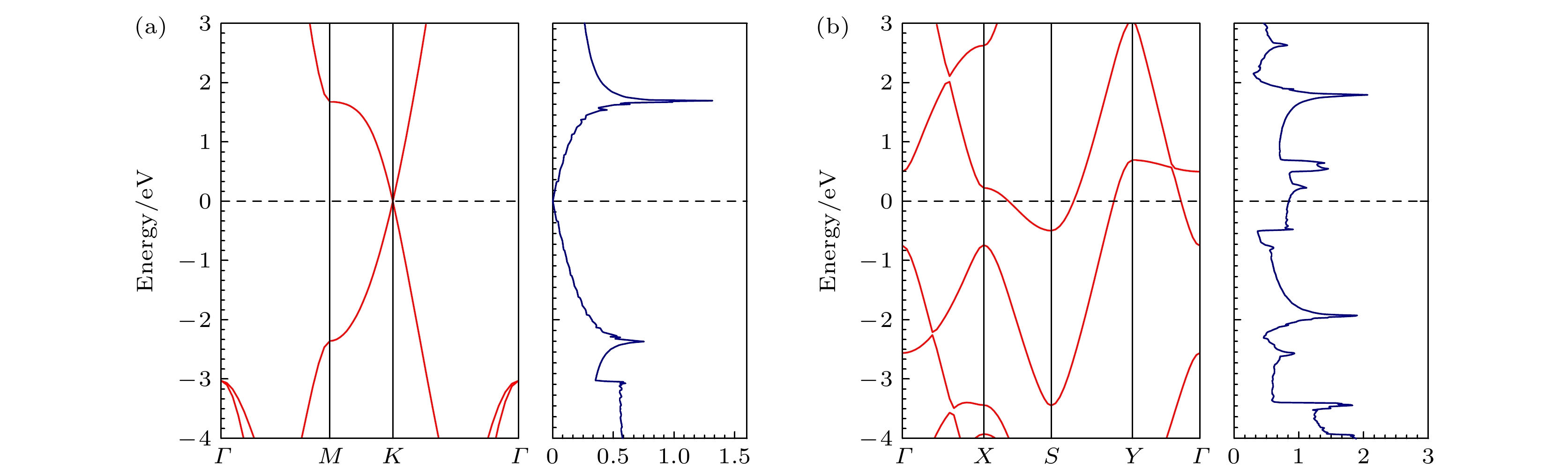
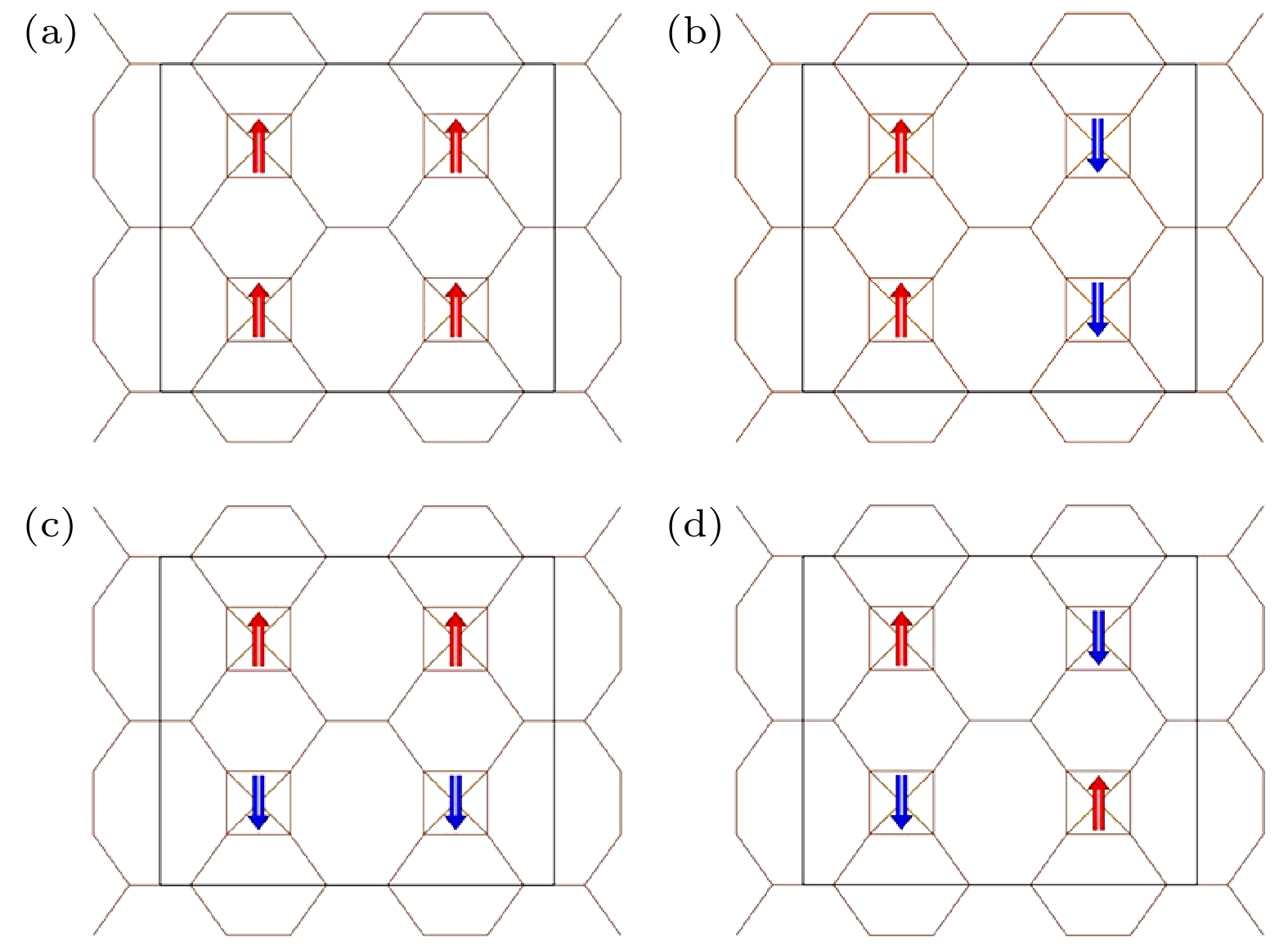
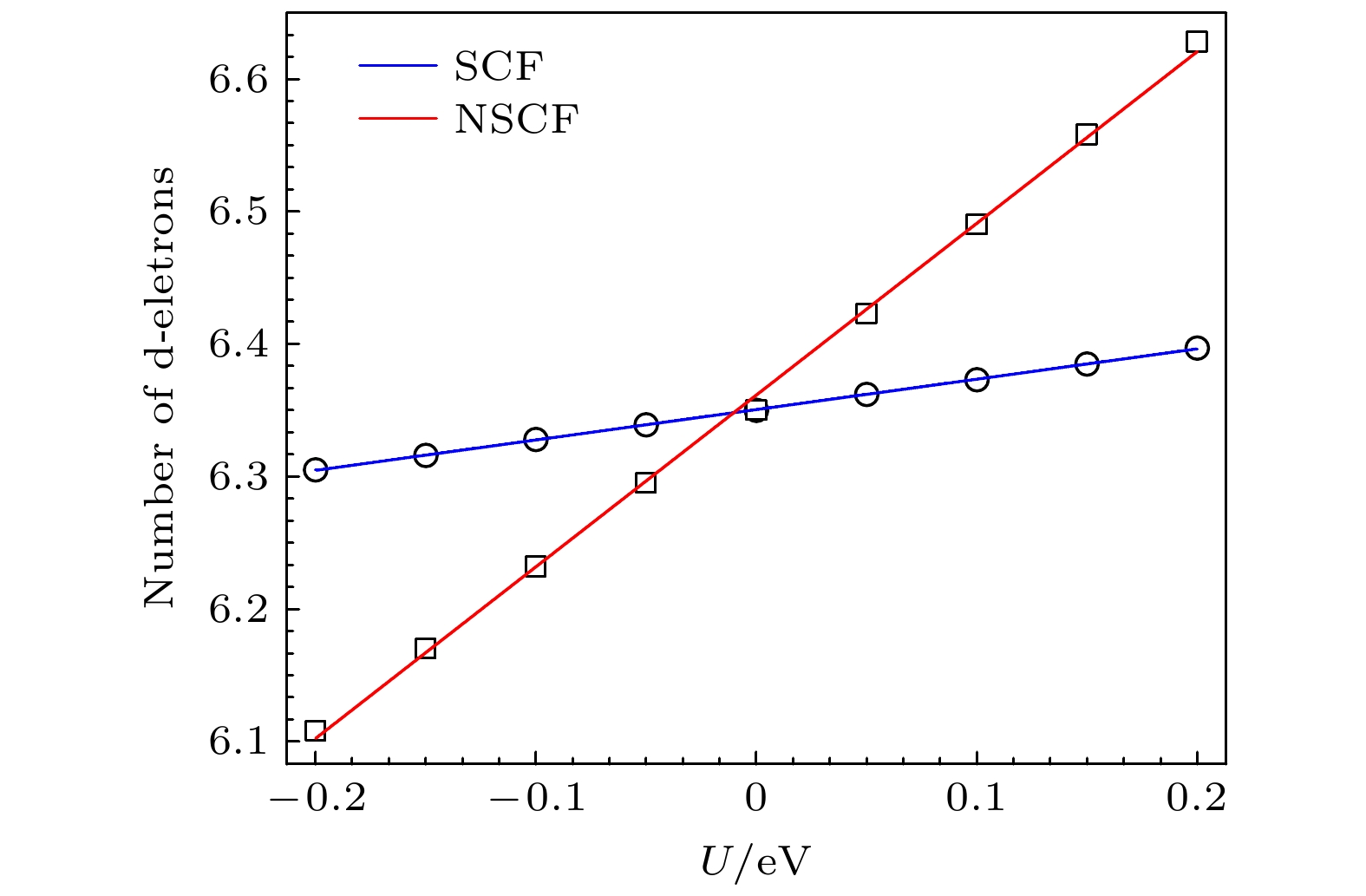
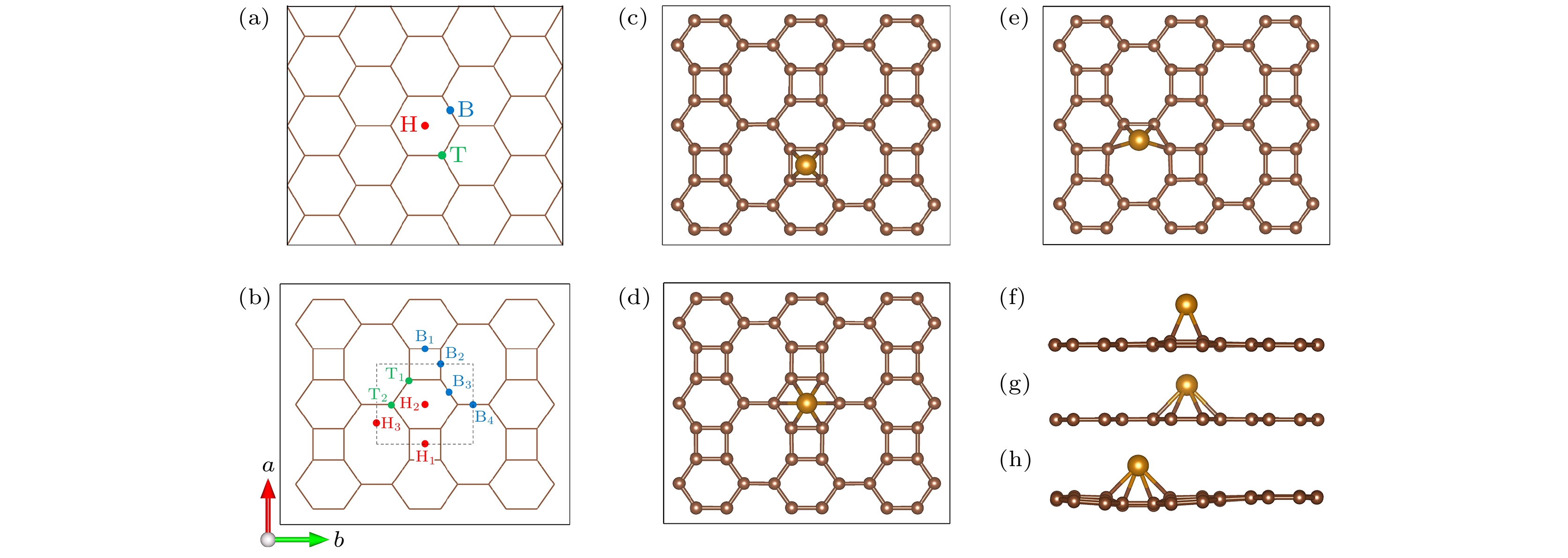
Biphenylene monolayer is composed of four-, six- and eight-membered carbon rings and has a monatomic layer structure similar to graphene. It was synthesized in experiment recently and reported in
Sciencein May 2021, which has attracted considerable attention in the research field of two-dimensional materials. By the density functional method of the first principle, we study the adsorption configuration of Fe atoms on biphenylene monolayer and analyze its electronic structure. The calculation of structural optimization, adsorption energy and molecular dynamics show that the biphenylene monolayer is a good matrix of Fe atoms. For Fe atoms, the hollow site in the four-membered ring of the biphenylene monolayer is the most stable adsorption site, and the adsorption energy can reach 1.56 eV. The calculation of charge transfer and density of states show that a stable bond can be formed between biphenylene monolayer and Fe atoms, and 0.73 electron is transferred from Fe atom to the neighbored carbon atom. After Fe atom being absorbed, biphenylene monolayer is magnetic, and the magnetic moment of Fe atom is about 1.81
${\mu}_{\mathrm{B}}$

and points out of the plane. Compared with graphene, biphenylene monolayer adsorbs Fe atoms more stably, which provides a new platform for studying the electromagnetic, transport and catalytic properties of two-dimensional materials with adatoms.














 下载:
下载: