-
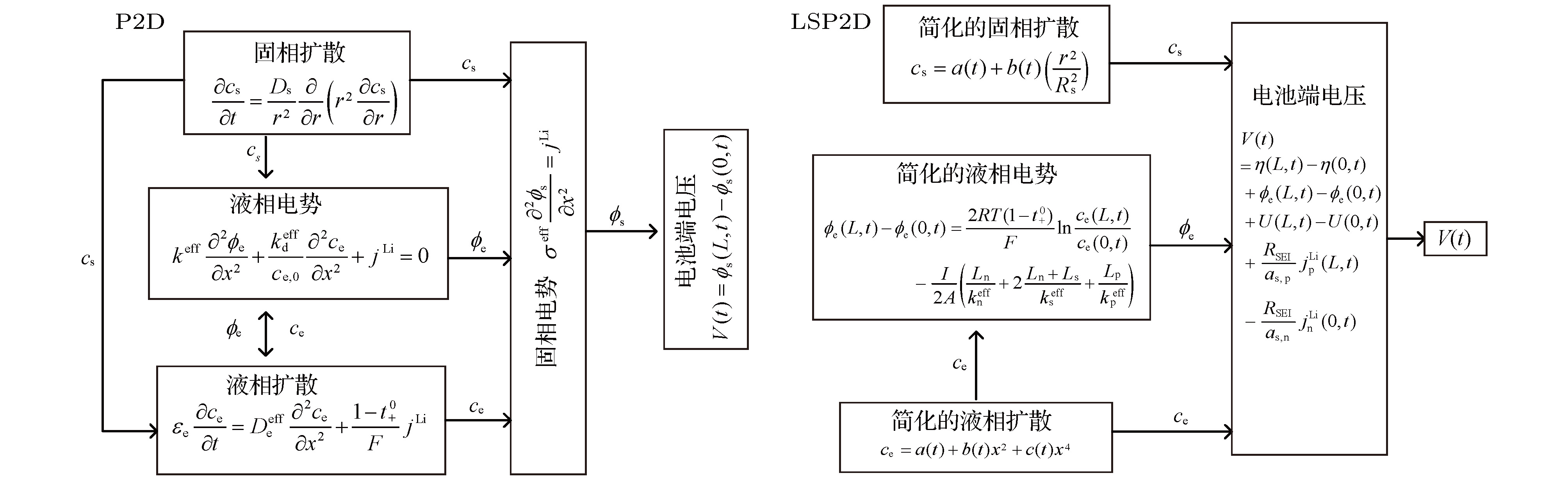
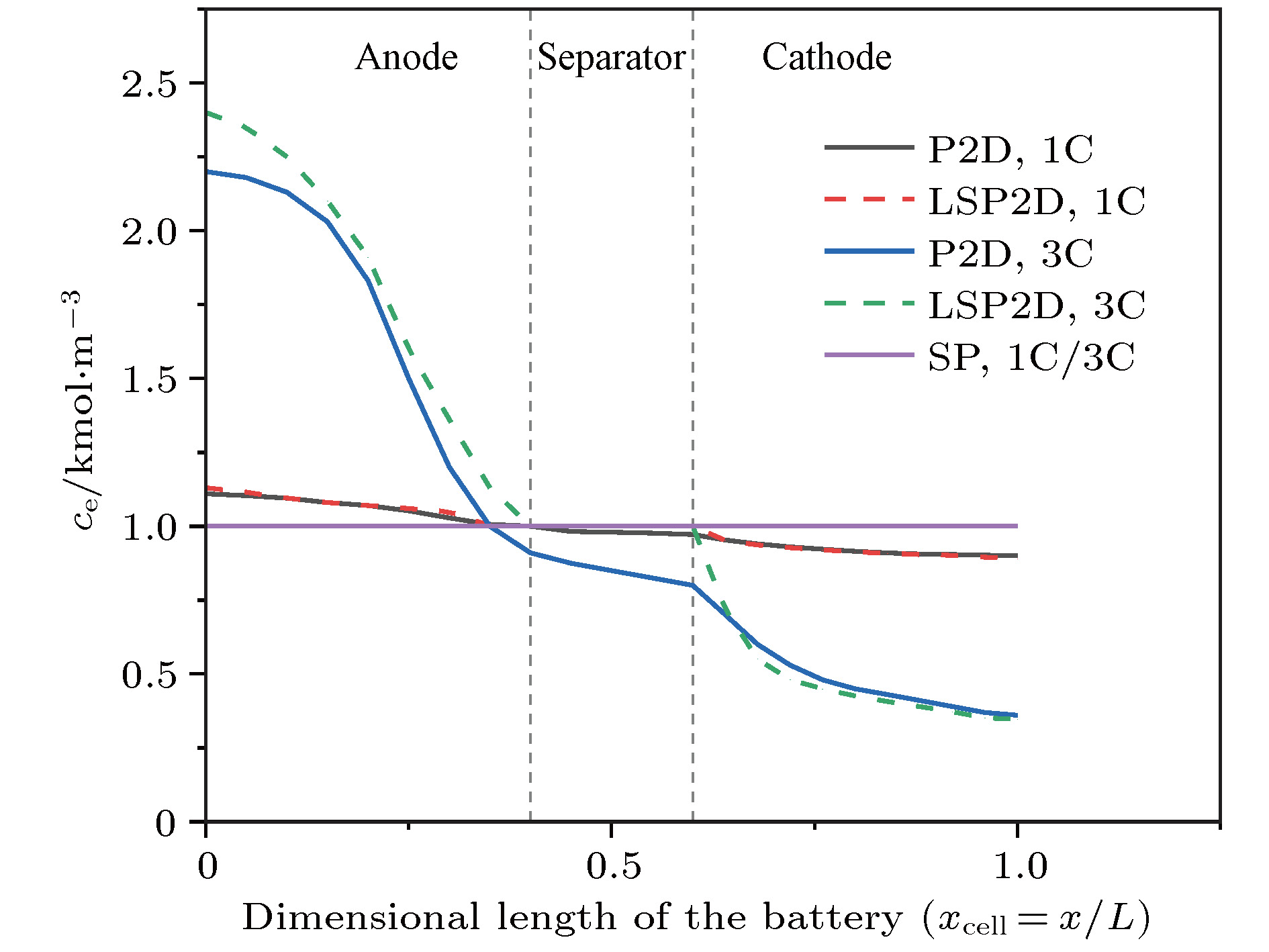
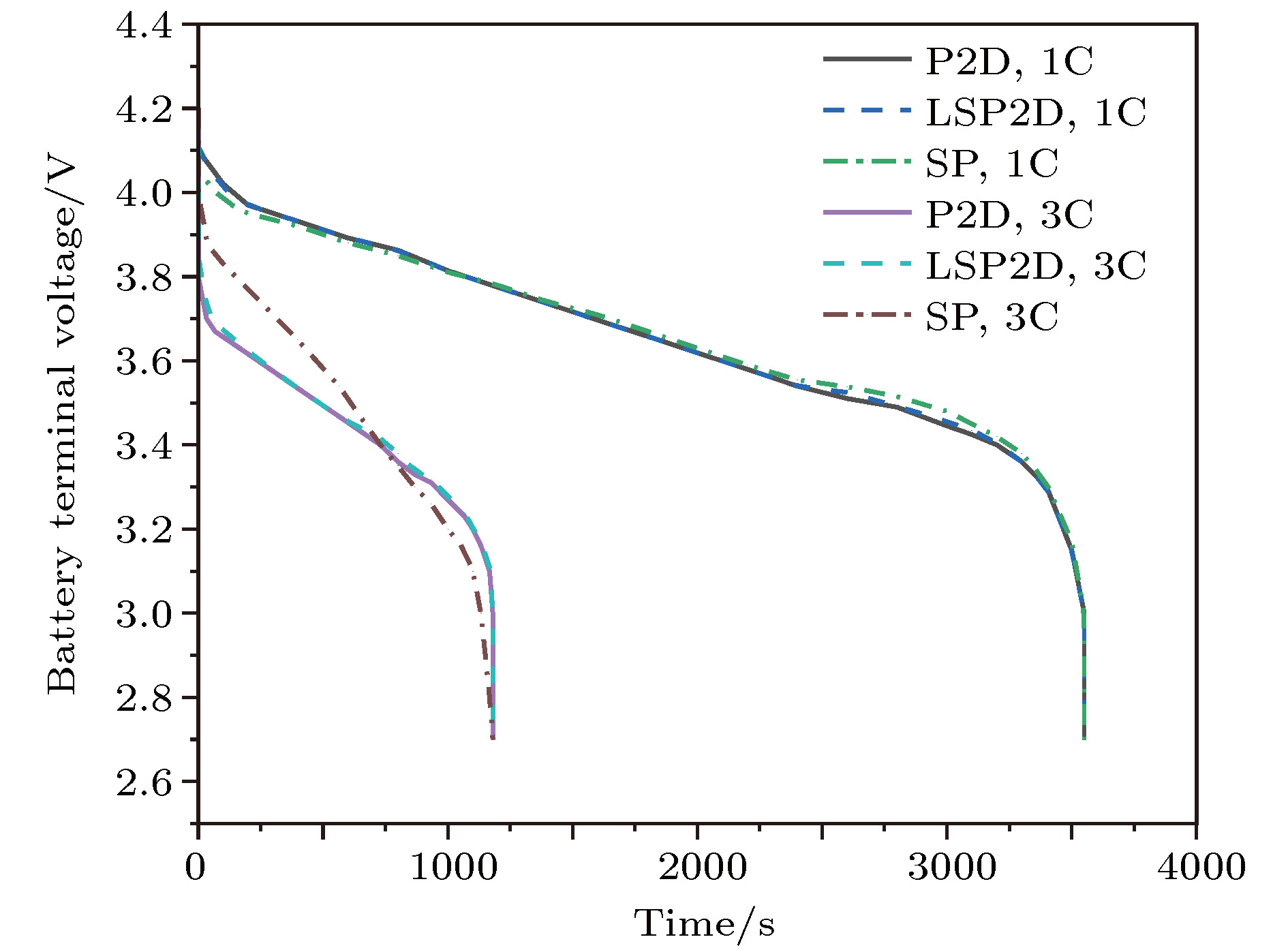
锂离子电池的电化学模型对于电池特性分析和电池管理具有重要意义, 但是准二维(P2D)模型复杂度太高, 为了在保证模型精度的基础上尽量降低复杂度, 本文提出了一种包含液相简化的P2D (LSP2D)模型, 该模型首先基于电化学平均动力学将电池端电压化简成为仅耦合固相Li +浓度 c s和液相Li +浓度 c e的方程, 然后进一步对表达 c s和 c e演化规律的偏微分方程进行抛物线近似化简, 使得最终的模型由多项式组成. COMSOL仿真表明在放电倍率为1C时该模型与单粒子(SP)模型的估算精度和速度相当, 但在放电倍率为3C时, 该模型的估算时间比P2D模型减少了99.73%, 与SP模型相当, 估算精度相比SP模型有大幅度提升.The pseudo-two-dimensional (P2D) model is the most widely used electrochemical model for lithium-ion batteries. Because of the complexity and the difficulty in using the complete P2D model, many simplified P2D models, such as the single particle model (SP model) and the parabolic profile approximation model (PP model), have been proposed. However, the using of the SP model can cause a large amount of precision to lose in its simplified process, while the PP model has a high complexity. In this paper, we propose a liquid phase simplification P2D (LSP2D) model. The using of the LSP2D model has a small precision loss and a relatively low complexity. The LSP2D model is based on the electrochemical average kinetics of the lithium ion battery. We first simplify the terminal voltage into an equation containing only the solid phase concentration c sand the liquid phase concentration c e. Then we use the partial differential equation to represent the solid phase concentration c sand the liquid phase concentration c e, and then obtain a final model. The simulation environment is based on COMSOL, and the simulation results show that when the discharge rate is 1C, the estimation accuracy and speed from the LSP2D model are similar to those from the SP model. But when the discharge rate is 3C, the estimation time from the LSP2D model is reduced by 99.73% compared with that from the P2D model, and the estimation accuracy is greatly improved compared with the estimation accuracy from the SP model.
-
Keywords:
- electrochemical model/
- battery management system/
- liquid phase diffusion equation/
- parabolic approximation
[1] [2] [3] [4] [5] [6] [7] [8] [9] [10] [11] [12] [13] [14] [15] [16] [17] [18] [19] [20] -
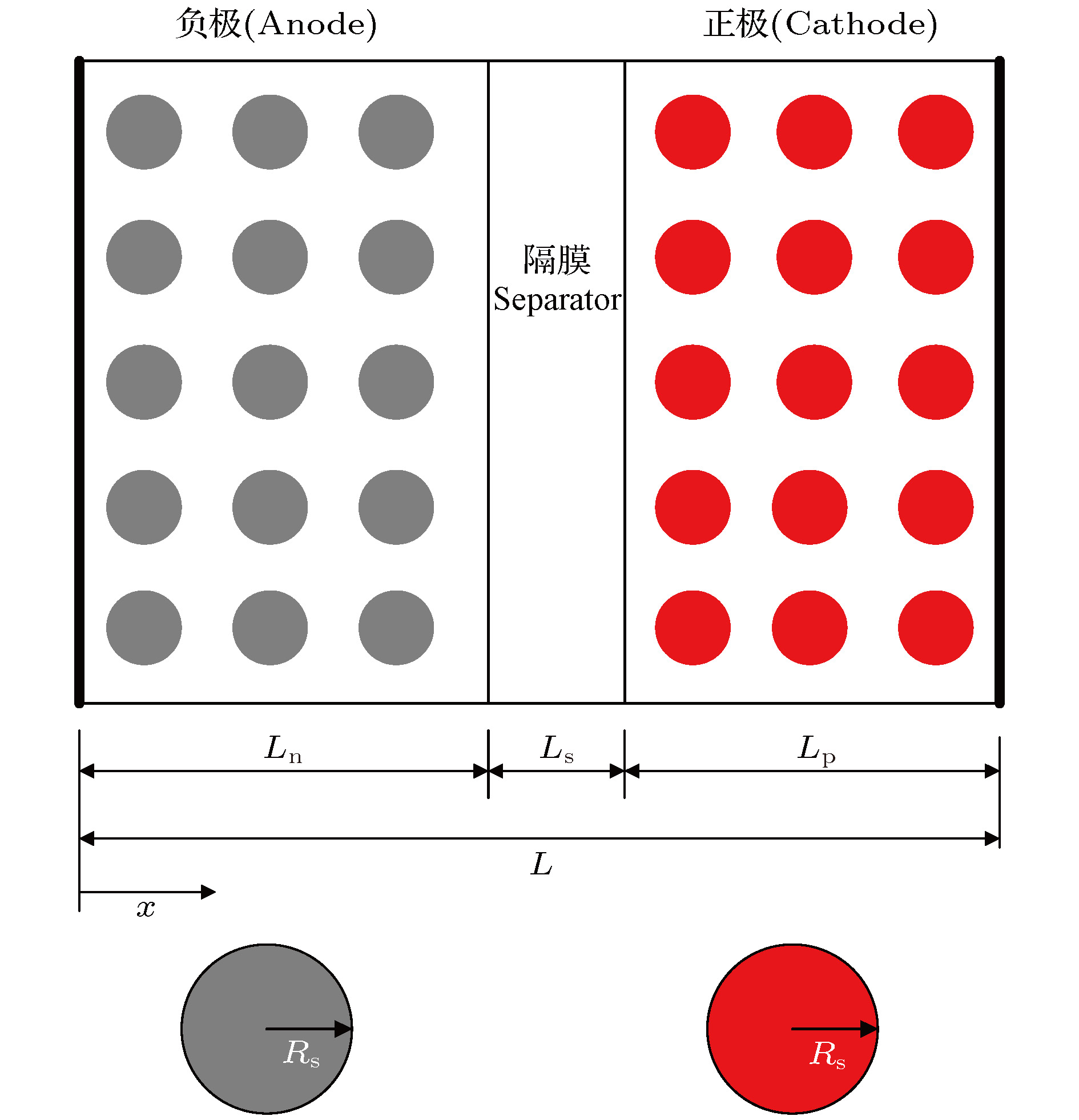
${a_1}$ ${b_1}$ ${c_1}$ ${a_2}$ ${b_2}$ ${a_3}$ ${b_3}$ ${c_3}$ $\displaystyle\frac{1}{8} + \alpha \tau $ $\displaystyle\frac{{15}}{4}$ $ - \displaystyle\frac{{15}}{8}$ $1$ 0 1 $\displaystyle\frac{{30}}{7}\beta \tau $ $ - \displaystyle\frac{{15}}{7}\beta \tau $ Symbol Anode Cathode Separator $\sigma$/S·m–1 100 100 ${\varepsilon _{\rm s}}$ 0.49 0.59 ${\varepsilon _{\rm e}}$ 0.485 0.365 0.724 Brug 4.0 4.0 4.0 ${c_{{\rm{e,0}}}}$/mol·m–3 1000 1000 1000 ${c_{{\rm{s,0}}}}$/mol·m–3 916.65 48977.25 ${c_{{\rm{s,max}}}}$/mol·m–3 30555 51555 A/m2 $6.03 \!\times\! {10^{ - 4}}$ $5.31 \!\times\! {10^{ - 4}}$ ${D_{\rm{e}}}$/m2·s–1 $7.5 \!\times\! {10^{ - 10}}$ $7.5 \!\times\! {10^{ - 10}}$ $7.5 \!\times\! {10^{ - 10}}$ ${D_{\rm{s}}}$/m2·s–1 $3.9 \!\times\! {10^{ - 14}}$ $1.0 \!\times\! {10^{ - 14}}$ k/mol·(mol·m–3)–1.5 $4.854 \!\times\! {10^{ - 6}}$ $2.252 \!\times\! {10^{ - 6}}$ ${R_{\rm{s}}}$/m $2 \!\times\! {10^{ - 6}}$ $2 \!\times\! {10^{ - 6}}$ x/m $8.8 \!\times\! {10^{ - 5}}$ $8.0 \!\times\! {10^{ - 5}}$ $8.0 \!\times\! {10^{ - 5}}$ ${R_{{\rm{SEI}}}}/\Omega$·m–2 0.01 I/A·m–2 20 $\alpha $ 0.5 0.5 F/C·mol 96487 R/J·mol·K–1 8.314 T/K 298.15 Model Average error at
discharge rate 1C/VAverage error at
discharge rate 3C/VP2D 0 0 LSP2D 0.0056 0.014 SP 0.0052 0.142 Model Time for 50 cycles/s P2D 7860 LSP2D 21 SP 12 -
[1] [2] [3] [4] [5] [6] [7] [8] [9] [10] [11] [12] [13] [14] [15] [16] [17] [18] [19] [20]
计量
- 文章访问数:12645
- PDF下载量:314
- 被引次数:0













 下载:
下载: